Rezatapopt is an investigational agent that has not been approved by any health authority.
PYNNACLE study
What is the PYNNACLE study?
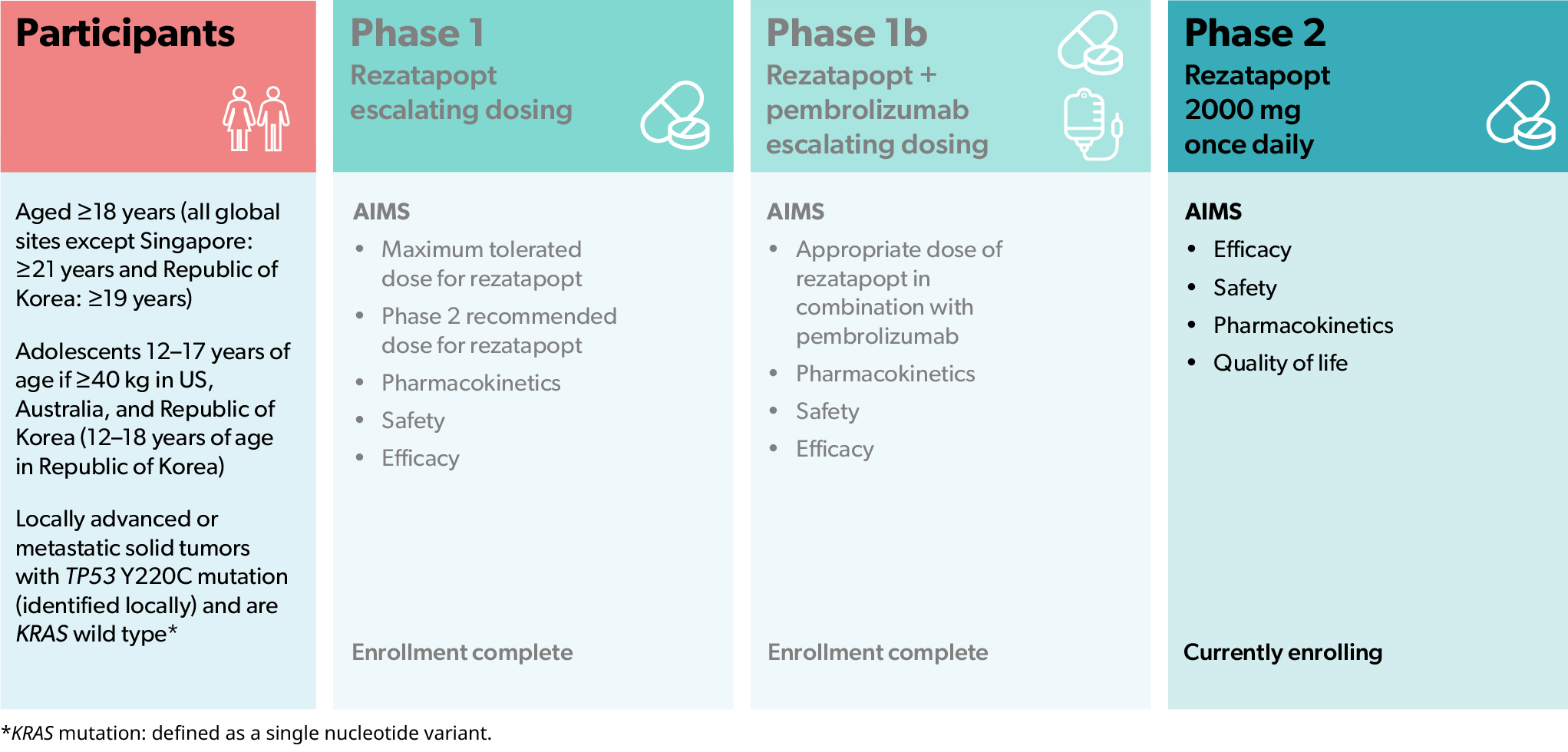
PYNNACLE (NCT04585750) is an ongoing Phase 1/2 clinical study of rezatapopt in patients with locally advanced or metastatic solid tumors that have a TP53 Y220C mutation1
Rezatapopt is a first-in-class, oral, selective p53 reactivator that specifically targets the p53 Y220C-mutated protein1–4
See below for more information on rezatapopt
What are the aims of the PYNNACLE study?
The aims of the PYNNACLE study are to assess the safety, tolerability, pharmacokinetics, pharmacodynamics, and efficacy of rezatapopt in patients with solid tumors that have a TP53 Y220C mutation, including patients with ovarian cancer, lung cancer, breast cancer, or endometrial cancer, as well as other types of cancer1
- The aims of the Phase 1 part of this study were to find a suitable dose of rezatapopt as well as assess pharmacokinetics, safety, and efficacy1
- Enrollment into the Phase 1 Monotherapy portion is complete1
- The aim of the Phase 1b portion of the study was to evaluate rezatapopt in combination with pembrolizumab1
- Enrollment into the Phase 1b Combination therapy portion is complete1
- The aims of the Phase 2 part of this study are to assess the efficacy of rezatapopt on its own as well as the safety, pharmacokinetics, and quality of life of participating patients1
- This part of the study is currently enrolling1
This study is designed to find out how best to treat certain locally advanced or metastatic solid tumors with the investigational drug rezatapopt. This study may be of no benefit to you. Taking part in this study may or may not improve your health. You may benefit from the study in that you may be seen for medical evaluation more frequently than you would be seen under the usual standard of care. No other benefits can be expected, but the information obtained from this study may benefit other patients with similar locally advanced or metastatic solid tumors.
Who is eligible to take part in the Phase 2 part of the PYNNACLE study?
The following eligibility criteria apply1
For more information on the eligibility criteria, please visit clinicaltrials.gov
Inclusion criteria:
Patients with a locally advanced or metastatic solid tumor that harbors a TP53 Y220C mutation
Aged ≥18 years (all global sites except Singapore: ≥21 years and Republic of Korea: ≥19 years)
Adolescents 12–17 years of age if ≥40 kg in US, Australia, and Republic of Korea (12–18 years of age in Republic of Korea).
Previously treated with one or more lines of anticancer therapy
Eastern Cooperative Oncology Group performance status score of 0 or 1
Exclusion criteria:
Brain metastases, unless neurologically stable
History of leptomeningeal disease, spinal cord compression, organ transplant, or gastrointestinal disease which may impact study drug absorption
Primary central nervous system tumor
Heart conditions (unstable angina within 6 months prior to screening, uncontrolled hypertension, heart attack within 6 months prior to screening, heart failure or other clinically significant rhythm abnormalities)
Known KRAS mutation, defined as a single nucleotide variant
Where is the PYNNACLE study taking place?
PYNNACLE is now enrolling patients with solid tumors that have a TP53 Y220C mutation at medical centers around the world
p53 Y220C and rezatapopt
What is the p53 protein, and what are the impacts of a TP53 gene mutation?
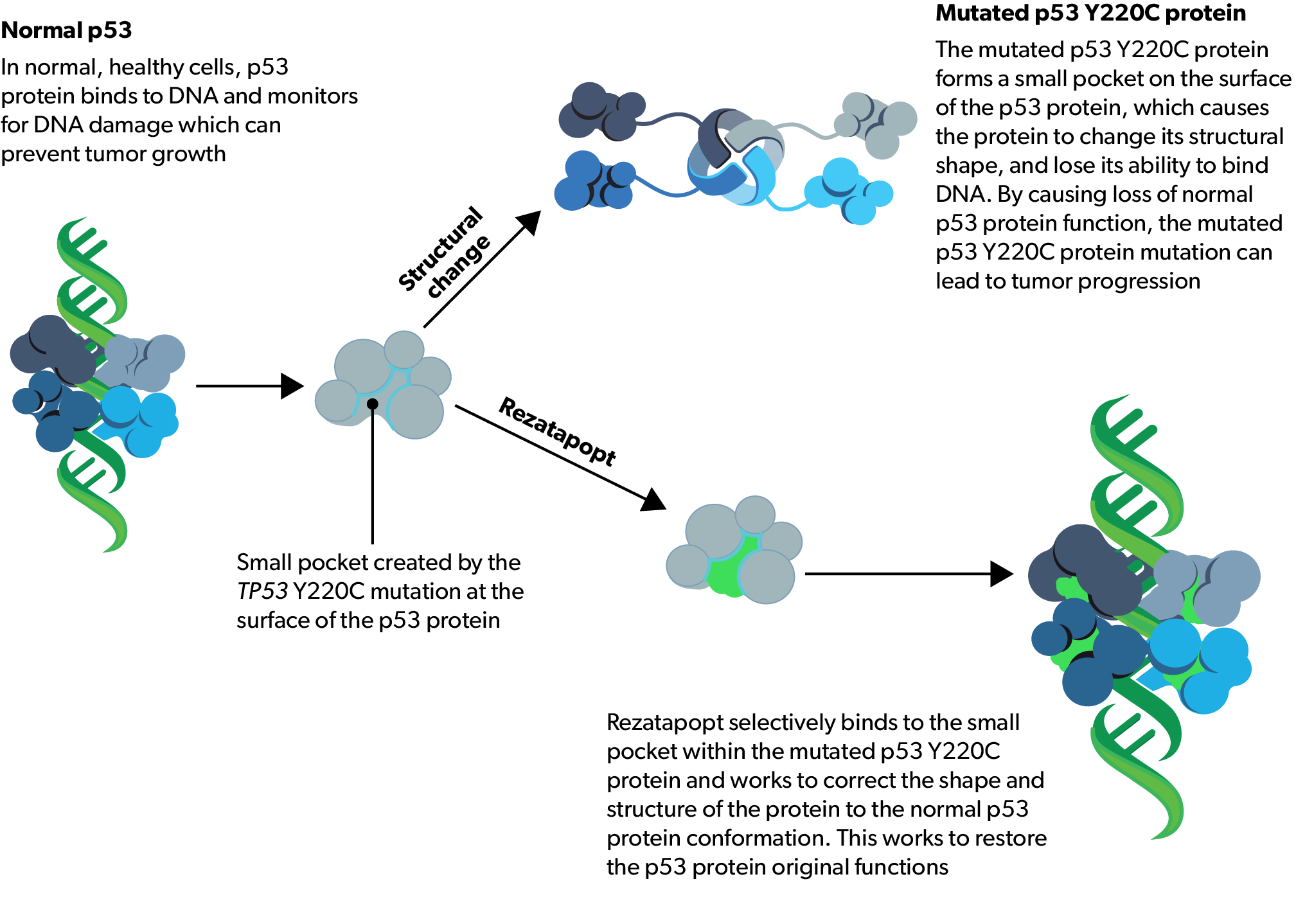
In normal, healthy cells, p53 is a tumor-suppressor protein that checks cells for damage to DNA and signs of stress and that can prevent tumors from getting worse (progressing)5,6
Mutations in the TP53 gene, which codes for the p53 protein, are commonly found in human cancers and can result in the loss or change of normal p53 protein function7–10
Mutated p53 protein has an important role in cancer cells by changing gene expression and causing tumor growth, survival, and progression5,9,11–19
What is the TP53 Y220C mutation?
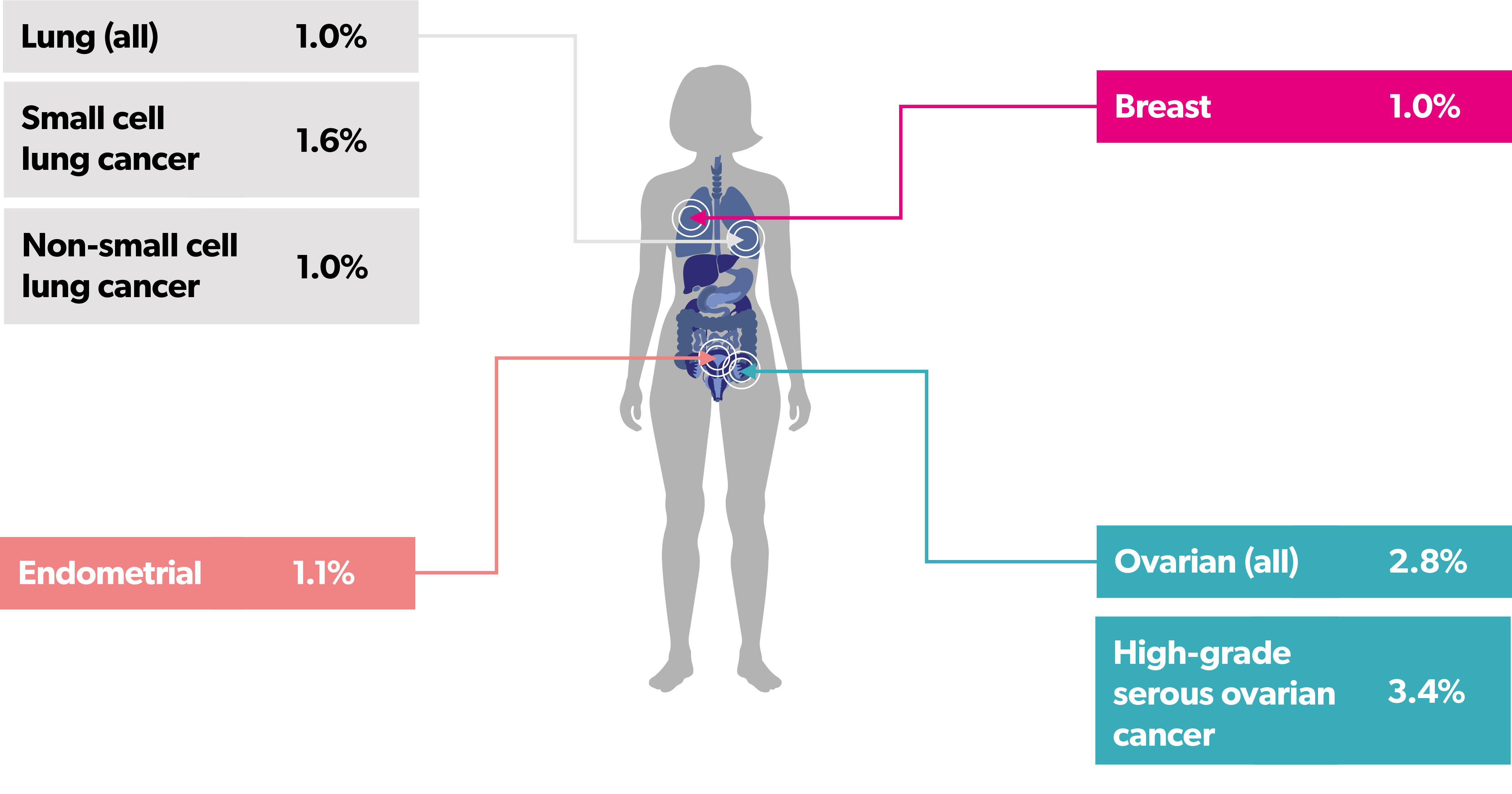
The TP53 Y220C mutation has been identified in over 30 different types of solid tumors4,14
People with ovarian cancer are most likely to have a TP53 Y220C mutation, followed by those with endometrial cancer, breast cancer, and lung cancer14
The TP53 Y220C mutation results in a small pocket forming on the surface of the p53 protein, which causes the protein to change shape and lose its ability to bind to DNA9,13,15–18
By causing a loss of normal p53 protein function, the mutated p53 Y220C protein can lead to tumor progression and is therefore a potential target for treating some cancers16–18
What is rezatapopt?
Rezatapopt is a first-in-class, oral, selective p53 reactivator that specifically targets the p53 Y220C-mutated protein1–4
Rezatapopt is currently under investigation for the treatment of patients with locally advanced or metastatic solid tumors that have a TP53 Y220C mutation (or TP53 Y220C cancer), including ovarian cancer, lung cancer, breast cancer, and endometrial cancer, as well as other types of cancer.1–4 For more information, view the PYNNACLE study section of this page or visit clinicaltrials.gov
Rezatapopt selectively binds to the small pocket within the p53 Y220C-mutated protein and works to correct the shape of the protein1–4
This restores p53 structure and function.1-4
Biomarker testing
What is precision medicine, and how is it used in cancer?
Cancer is caused when abnormalities in DNA (for example, mutations) lead to changes in the function of a gene, resulting in uncontrollable growth and survival of tumor cells19
Learn more about genes and cancer with the American Cancer Society
Key information about a person’s specific cancer can be found by testing for cancer biomarkers, which include genes, proteins, and other substances20
The comprehensive biomarker profile of a tumor can be used to make a diagnosis, to decide treatment options based on gene mutations, and to assess how well a treatment is working – this is called precision medicine20,21
Biomarker testing provides a molecular profile of a tumor, which may give information on whether an approved therapy or clinical study could be an option20,21
Learn more about precision medicine and biomarker testing with the American Cancer Society
Next-generation sequencing (NGS) is a method of biomarker testing that identifies any genomic mutations and can provide important information to determine available treatments or clinical study options.22 Tissue from a patient’s tumor that has been removed either by biopsy or surgery can be assessed by NGS.22 For some patients, a blood sample may be used for NGS testing.22 NGS can provide important information to determine available treatments or clinical study options22
To learn more about next-generation sequencing, please visit JAMA Oncology
The TP53 Y220C mutation can be identified by NGS3
For more information, view the p53 Y220C and rezatapopt section of this page
Further information on clinical studies
Learn more about clinical studies with the National Cancer Institute, American Cancer Society, and European Medicines Agency
If you are interested in taking part in a clinical trial listed on clinicaltrials.gov, the Leal Health service can help find the right trial for you, for free. Click here to get started or visit the frequently asked questions, patient support resources, and questions to ask your oncologist pages for more information
To learn more about rezatapopt in the PYNNACLE study, please visit clinicaltrials.gov
Glossary
Biomarker testing
The use of a sample of body tissue (such as a tissue biopsy) or blood in a laboratory to check for particular genes, proteins, or other substances that might be a sign of cancer. Biomarker testing can help with making a diagnosis, assessing whether someone is at an increased risk of developing a certain type of cancer, planning treatment, seeing if a treatment is working, making a prognosis, and predicting if cancer might come back or spread elsewhere in the body23
Clinical study
Also called a clinical trial, this is a type of research study that investigates how effective a new medical approach (including screening, prevention, diagnosis, and treatment) is in humans23
Eastern Cooperative Oncology Group (ECOG) performance status score
A measurement of how well a person can function and take care of themselves24
Efficacy
How well a drug works and achieves the desired benefit23
Escalating dosing
Increasing the dose of a study drug by small amounts to ensure it is tolerated or to increase its clinical effect25
KRAS gene
The gene that encodes the KRAS protein, which has important roles in controlling cell growth, maturation, and death. Mutations in the KRAS gene can be found in some cancer types and may help cancer cells to grow and spread to other locations in the body23
Locally advanced cancer
Cancer that has spread to lymph nodes or tissue near where it began in the body23
Measurable disease
A tumor that can be precisely measured, which can help to evaluate how well the cancer is responding to treatment23
Metastatic cancer
Cancer that has spread from where it started and formed new tumors elsewhere in the body23
Mutation
A change to the DNA of a cell. These changes can be introduced by mistakes when a cell divides and multiplies, or from contact with things that can damage DNA such as toxic chemicals, radiation, or ultraviolet rays like those from sunlight23,26
Next-generation sequencing
A method using computers to assess millions of DNA fragments, taken from cells in a sample of tissue, at the same time. Next-generation sequencing can detect changes in specific genes and can help researchers and healthcare professionals determine what has caused the cancer23
Oral
A medicine that is taken by mouth (ie, swallowing a pill)23
Pharmacodynamics
The study of how a drug behaves in the body, such as which receptors it binds to, how strongly it binds, effects that occur after binding, and chemical interactions27
Pharmacokinetics
The study of what the body does to a drug over time, such as how it is absorbed and moved around the body, where it localizes (in which tissues/organs), and how it is removed from the body23
Precision medicine
Preventing, diagnosing, or treating disease by using information about someone’s genes, proteins, environment, and lifestyle. In cancer, knowledge about a person’s tumor can help with making a diagnosis, assessing if they are at an increased risk of developing a certain type of cancer, planning treatment, seeing if a treatment is working, making a prognosis, or predicting if cancer might come back or spread elsewhere in the body23
Progression of disease
Cancer becoming worse or spreading elsewhere in the body23
PYNNACLE
An ongoing Phase 1/2 clinical study of rezatapopt in patients with locally advanced or metastatic solid tumors that have a TP53 Y220C mutation (NCT04585750).1 For more information, view the PYNNACLE study section of this page or visit clinicaltrials.gov
Quality of life
An assessment of how much someone enjoys life, their sense of well-being, and their ability to perform tasks of daily living23
Rezatapopt
A first-in-class, oral, selective p53 reactivator that specifically targets the p53 Y220C-mutated protein.1–4 For more information, view the rezatapopt section of this page
Safety
An assessment of risks of a drug being tested in a clinical study, using laboratory tests, specific safety tests (eg, electrocardiograms), changes in vital signs (basic bodily functions such as pulse, breathing rate, body temperature, and blood pressure), and incidence of diseases, signs, and symptoms28–30
Single nucleotide variant
A type of mutation in which only one nucleotide (a building block of DNA) is swapped with a different kind23
Tolerability
The determination of the severity of side effects of a drug that can be withstood by a patient28,29
TP53 gene
A gene that encodes the tumor suppressor p53 protein, which plays a key role in controlling cell division and death. If the TP53 gene becomes mutated, it may result in cancer cells being able to grow and spread elsewhere in the body23
Tumor suppressor protein
Proteins that are key in controlling cell growth. Mutations in the genes of tumor suppressor proteins may result in the development of cancer23
Wild type
Genes in their original, natural, unchanged (non-mutated) form23
References
1. ClinicalTrials. gov. NCT04585750. The evaluation of PC14586 in patients with advanced solid tumors harboring a p53 Y220C mutation. Available at: https://clinicaltrials.gov/study/NCT04585750. Accessed October 2025; 2. Dumble M, et al. Oral presentation at AACR 2021, Virtual. April 10–15, 2021; 3. Dumbrava EE, et al. Oral presentation at ASCO 2022, Chicago, USA. June 3–7, 2022; 4. Puzio-Kuter AM, et al Restoration of the Tumor Suppressor Function of Y220C-Mutant p53 by Rezatapopt, a Small-Molecule Reactivator. Cancer Discov 1 June 2025; 15 (6): 1159–1179; 5. Kastenhuber ER, et al. Cell. 2017;170:1062–1078; 6. Blagih J, et al. J Cell Sci. 2020;133:jcs237453; 7. Levine AJ. Ann Rev Cancer Biol. 2019;3:21–34; 8. Donehower LA, et al. Cell Rep. 2019;28:1370–1384.e1375; 9. Baugh EH, et al. Cell Death Differ. 2018;25:154–160; 10. Yue X, et al. J Mol Biol. 2017;429:1595–1606; 11. Alexandrova EM, et al. Cell Death Dis. 2017;8:e2661; 12. Mantovani F, et al. Cell Death Differ. 2019;26:199–212; 13. Roszkowska KA, et al. Int J Mol Sci. 2020;21:1334; 14. FoundationInsights™. A proprietary database used under license with review and approval from Foundation Medicine®. Available at: https://www.foundationmedicine.com/service/genomic-data-solutions. Accessed October 2025; 15. Bouaoun L, et al. Hum Mutat. 2016;37:865–876; 16. Blanden AR, et al. Drug Discov Today. 2015;20:1391–1397; 17. Baud MGJ, et al. Eur J Med Chem. 2018;152:101–114; 18. Tang Y, et al. J Phys Chem B. 2021;125:10138–10148; 19. American Cancer Society. Genes and cancer. Available at: https://www.cancer.org/cancer/understanding-cancer/genes-and-cancer.html. Accessed October 2025; 20. American Cancer Society. Biomarker tests and cancer treatment. Available at: https://www.cancer.org/cancer/diagnosis-staging/tests/biomarker-tests.html. Accessed October 2025; 21. American Cancer Society. Precision or personalized medicine. Available at: https://www.cancer.org/cancer/managing-cancer/treatment-types/precision-medicine.html. Accessed October 2025; 22. Ewalt MD, et al. JAMA Oncol. 2019;5:1076; 23. National Cancer Institute. NCI dictionary of cancer terms. Available at: https://www.cancer.gov/publications/dictionaries/cancer-terms. Accessed October 2025; 24. VeryWellHealth. What is performance status? Available at: https://www.verywellhealth.com/what-is-performance-status-2249416. Accessed October 2025; 25. Yap C, et al. BMJ. 2023;383:e076387; 26. Chatterjee N & Walker GC. Environ Mol Mutagen. 2017;58:235–263; 27. Farinde A. Overview of pharmacodynamics. Available at: https://www.msdmanuals.com/professional/clinical-pharmacology/pharmacodynamics/overview-of-pharmacodynamics. Accessed October 2025; 28. US CDER CBER. Guidance for industry E9 statistical principles for clinical trials. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e9-statistical-principles-clinical-trials. Accessed October 2025; 29. EMA. ICH E9 statistical principles for clinical trials - scientific guideline. Available at: https://www.ema.europa.eu/en/ich-e9-statistical-principles-clinical-trials-scientific-guideline. Accessed October 2025; 30. Cleveland Clinic. Vital signs. Available at: https://my.clevelandclinic.org/health/articles/10881-vital-signs. Accessed October 2025.
Please contact the PMV Pharmaceuticals Clinical Study Information Center
+1 (609) 235-4038
clinicaltrials@pmvpharma.com
Rezatapopt is an investigational agent that has not been approved by any health authority.
MA-586-0107 October 2025
