The TP53 Y220C mutation has been identified in many types of solid tumors
The TP53 Y220C mutation is a potential treatment target in cancer and has been identified in over 30 different solid tumor types.1–3 Under normal conditions, p53 is a tumor-suppressor protein and has a key role in disrupting tumor progression.4,5 The mutated p53 Y220C protein forms a small pocket at the surface of p53 protein, which causes the protein to lose its structural shape; this results in a loss of function, and can lead to tumor progression.6,7
References: 1. Dumbrava EE, et al. Oral presentation at ASCO 2022, Chicago, USA. June 3–7, 2022; 2. Schram A, et al. Oral presentation at AACR-NCI-EORTC 2023, Boston, USA. October 11–15, 2023; 3. Bauer M, et al. Future Med Chem. 2019;11(19):2491–2504; 4. Chillemi G, et al. Cold Spring Harb Perspect Med. 2017;7:a028308; 5. Kastenhuber ER, et al. Cell. 2017;170:1062–1078; 6. Baugh EH, et al. Cell Death Differ. 2018;25:154–160; 7. Baud MGJ, et al. Eur J Med Chem. 2018;152:101–114.
Rezatapopt is being investigated in TP53 Y220C cancer
Rezatapopt is a first-in-class, selective p53 reactivator specific to the mutated p53 Y220C protein.1,2 Rezatapopt is currently under investigation for the treatment of patients with locally advanced or metastatic solid tumors that have a TP53 Y220C mutation (or TP53 Y220C cancer), including but not limited to ovarian cancer, lung cancer, breast cancer and endometrial cancer.3-5
References: 1. The Evaluation of PC14586 in Patients With Advanced Solid Tumors Harboring a p53 Y220C Mutation. Available at clinicaltrials.gov. Accessed January 2024; 2. Joerger AC, et al. Proc Natl Acad Sci USA. 2006; 103:15056-15061; 3. Dumble M, et al. Cancer Res. 2021;81:Abstract LB006; 4. Dumbrava EE, et al. Oral presentation at ASCO 2022, Chicago, USA. June 3–7, 2022; 5. Schram A, et al. Oral presentation at AACR-NCI-EORTC 2023, Boston, USA. October 11–15, 2023.
Rezatapopt selectively reactivates p53 Y220C
Rezatapopt selectively binds to the small pocket within the TP53 Y220C mutated protein and corrects the shape and structure of the protein to normal p53 protein conformation.1–4 This restores the p53 protein original functions, including the ability to disrupt tumor progression.1–4
Rezatapopt mechanism of action


References: 1. Dumbrava EE, et al. Oral presentation at ASCO 2022, Chicago, USA. June 3–7, 2022; 2. Schram A, et al. Oral presentation at AACR-NCI-EORTC 2023, Boston, USA. October 11–15, 2023; 3. The Evaluation of PC14586 in Patients With Advanced Solid Tumors Harboring a p53 Y220C Mutation. Available at clinicaltrials.gov. Accessed January 2024; 4. Joerger AC, et al. Proc Natl Acad Sci U S A. 2006;103:15056–15061.
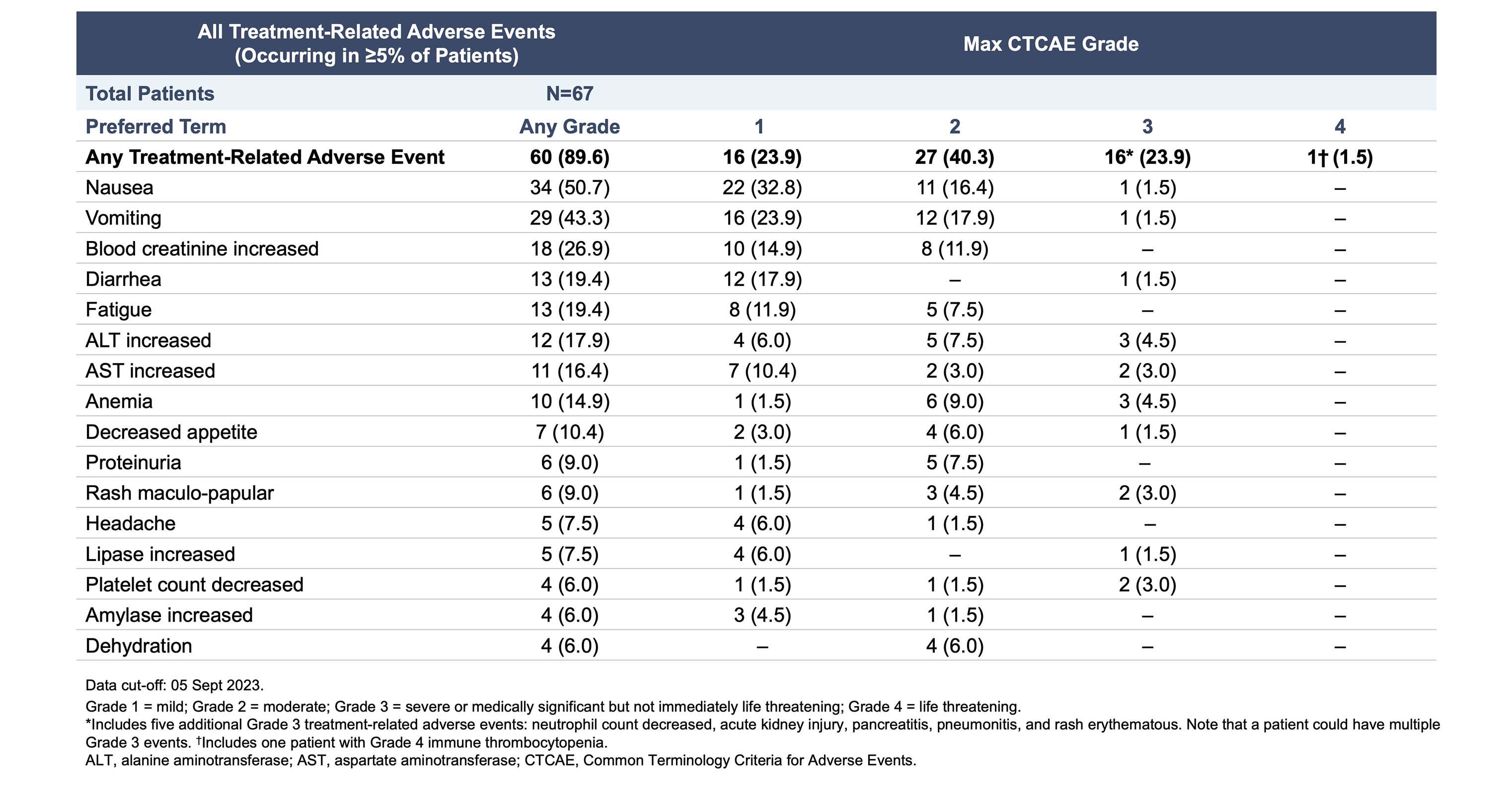
Data suggest rezatapopt is well-tolerated and reduces tumor size across a range of tumor types
These data showed that rezatapopt has an acceptable safety profile consisting of primarily mild or moderate events in patients with solid tumors that have a TP53 Y220C mutation
Rezatapopt treatment led to reduction in tumor size across a range of tumor types that harbor a TP53 Y220C mutation
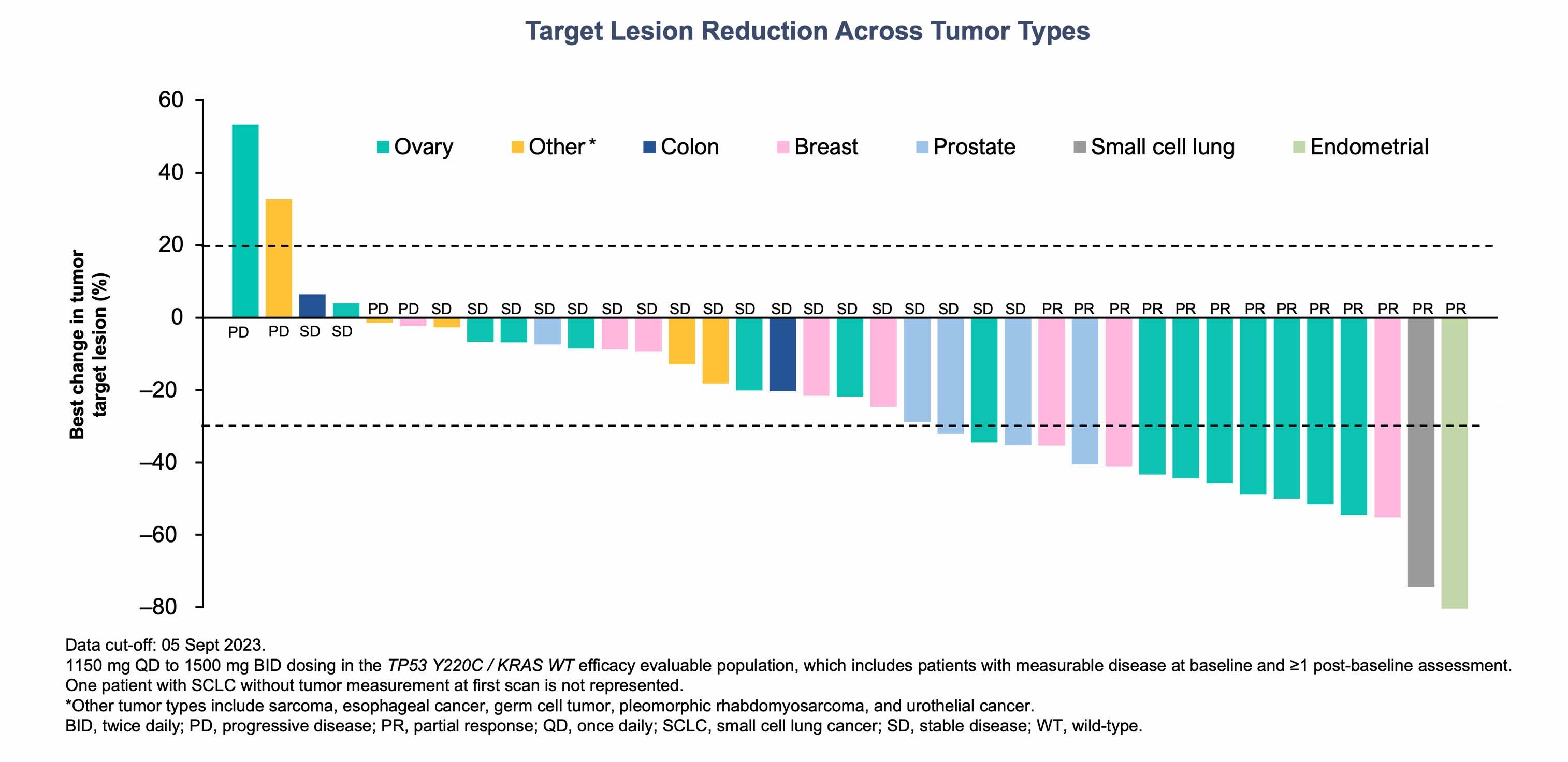
Reference: 1. Schram A, et al. Oral presentation at AACR-NCI-EORTC 2023, Boston, USA. October 11–15, 2023.
Biomarker testing can identify TP53 Y220C cancer
Biomarker testing can identify any genomic abnormalities in a tumor profile, including the TP53 Y220C mutation. This may provide information on whether an approved therapy or clinical study, such as the PYNNACLE study, could be an option.
Talk with your healthcare provider about whether biomarker testing is right for you.
Reference: 1. American Cancer Society. Biomarker Tests and Cancer Treatment. Accessed January 2024.
Presentations and Publications
Further information on clinical studies
Learn more about clinical studies with the National Cancer Institute, American Cancer Society, and European Medicines Agency.
If you are interested in taking part in a clinical trial, the Leal Health service can help find the right trial for you, for free. Click here for more information.
To learn more about rezatapopt (also known as PC14586) in the PYNNACLE study, please visit clinicaltrials.gov.
Please contact the PMV Pharmaceuticals Clinical Study Information Center
+1 (609) 235-4038
clinicaltrials@pmvpharma.com
Medical Information Request Form
For healthcare providers as well as patients and their caregivers to query PMV Pharmaceuticals for additional Medical Information.

Click here or scan the QR code for the Medical Information Request Form
Rezatapopt is an investigational agent that has not been approved by the US FDA, EMA or any other regulatory agency for the treatment of cancer.
MA-586-0014 February 2024